“Standard” and custom programmes for every need. he equipment process is developed by our Automation Department, according to the current codes/standards and the type of product being processed. Vacuum breaker or pressure leak tests; load conditioning programme; vacuum sterilisation programme; product degassing programme; and a gas neutralising programme, etc. The ETO gas is injected at a temperature of 60°C into the chamber via a PID-controlled modulating valve and the condensate is continuously evacuated through the drain, in order to ensure excellent heat distribution during the entire sterilisation phase (temperature deviation below ± 1°C).
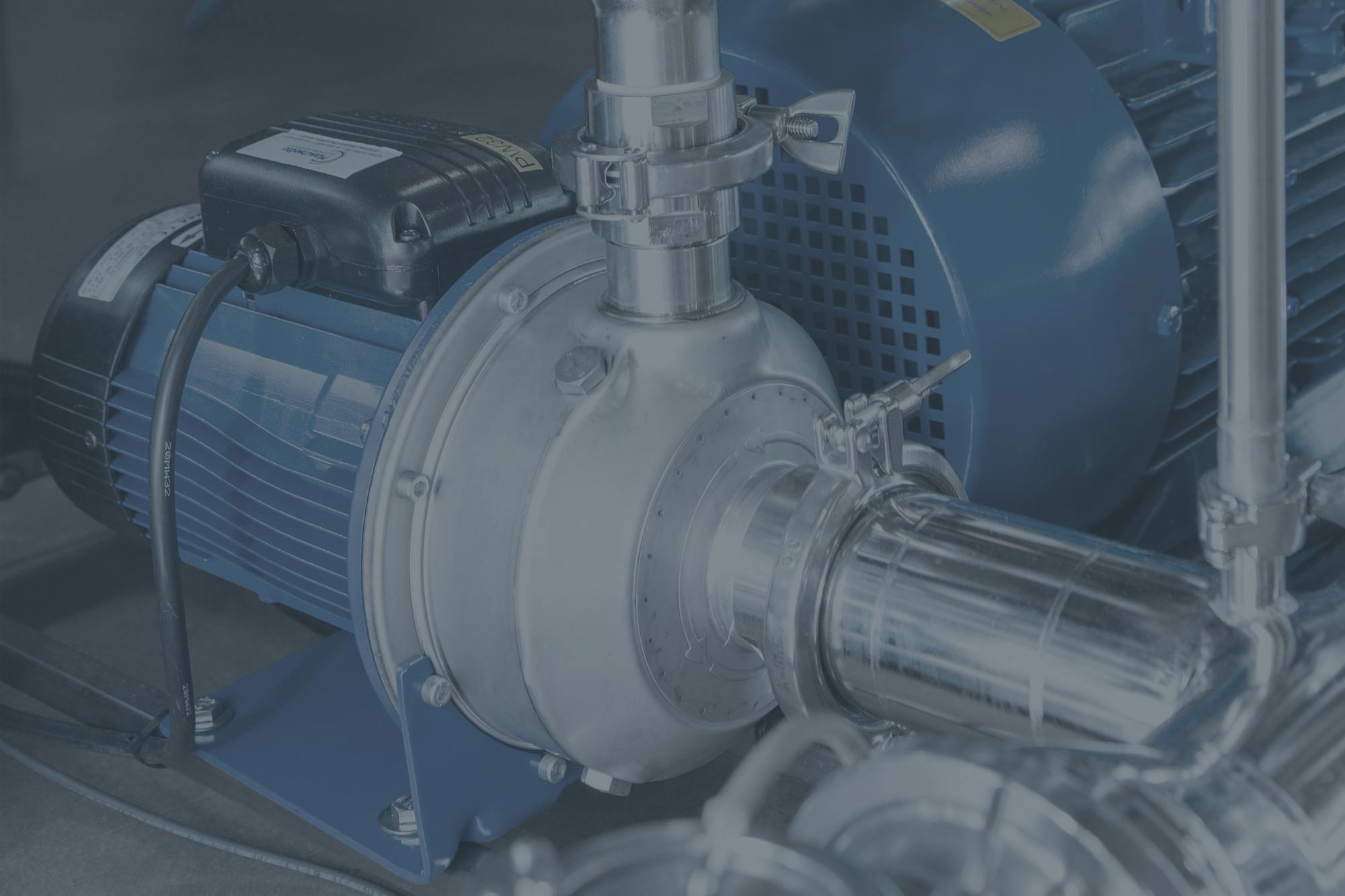
- Double-walled square chamber, made entirely from 316L or 316Ti stainless steel.
- Ashlar-type total cavity comprising 304 or 316L/316Ti stainless steel.
- Piping made entirely from 316L stainless steel with sanitary
- fittings (tri-clamp ferrules and hygienic flanges).
- Any surfaces that come into contact with the product and process fluids are mechanically polished to a roughness level of less than 0.35 micron.
- Automatic horizontal sliding chamber doors.
- Pneumatically pressurised chamber door sealing gasket (cwith process air).
- 316L/316Ti stainless steel components and tools, in addition to elastomers manufactured in compliance with FDA 21 CFR, Part 177.
- The chamber, heads, pipes, components and instruments are properly insulated by a cutting-edge material.
- Built-in steam generator.
- Built-in vacuum pump with a water recirculation system (water-saving system).
- Built-in ETO gas evaporation system.
- Built-in load conditioning, sterilising and degassing devices.
- Manual or fully automated ergonomic product loading and unloading solutions.
- Gas neutralisation system by means of a thermal combustor or scrubber.
- Floor or elevated loading solutions.
cGMP ethylene oxide sterilisers
 PHARMA DIVISION
PHARMA DIVISION
cGMP ethylene oxide sterilisers
The ETO sterilisers have been designed to sterilise – by means of ethylene oxide – heat-sensitive materials, such as plastic syringes, infusion sets, dialysis filter cartridges, plastic materials and special surgical tools.
![]() POROUS AND NON-POROUS
POROUS AND NON-POROUS
![]() ETHYLENE OXIDE + NITROGEN
ETHYLENE OXIDE + NITROGEN
![]() 20°C – 50°C
20°C – 50°C

“Standard” and custom programmes for every need. he equipment process is developed by our Automation Department, according to the current codes/standards and the type of product being processed. Vacuum breaker or pressure leak tests; load conditioning programme; vacuum sterilisation programme; product degassing programme; and a gas neutralising programme, etc. The ETO gas is injected at a temperature of 60°C into the chamber via a PID-controlled modulating valve and the condensate is continuously evacuated through the drain, in order to ensure excellent heat distribution during the entire sterilisation phase (temperature deviation below ± 1°C).
- Double-walled square chamber, made entirely from 316L or 316Ti stainless steel.
- Ashlar-type total cavity comprising 304 or 316L/316Ti stainless steel.
- Piping made entirely from 316L stainless steel with sanitary
- fittings (tri-clamp ferrules and hygienic flanges).
- Any surfaces that come into contact with the product and process fluids are mechanically polished to a roughness level of less than 0.35 micron.
- Automatic horizontal sliding chamber doors.
- Pneumatically pressurised chamber door sealing gasket (cwith process air).
- 316L/316Ti stainless steel components and tools, in addition to elastomers manufactured in compliance with FDA 21 CFR, Part 177.
- The chamber, heads, pipes, components and instruments are properly insulated by a cutting-edge material.
- Built-in steam generator.
- Built-in vacuum pump with a water recirculation system (water-saving system).
- Built-in ETO gas evaporation system.
- Built-in load conditioning, sterilising and degassing devices.
- Manual or fully automated ergonomic product loading and unloading solutions.
- Gas neutralisation system by means of a thermal combustor or scrubber.
- Floor or elevated loading solutions.
cGMP ethylene oxide sterilisers
 PHARMA DIVISION
PHARMA DIVISION
cGMP ethylene oxide sterilisers
The ETO sterilisers have been designed to sterilise – by means of ethylene oxide – heat-sensitive materials, such as plastic syringes, infusion sets, dialysis filter cartridges, plastic materials and special surgical tools.
![]() POROUS AND NON-POROUS
POROUS AND NON-POROUS
![]() ETHYLENE OXIDE + NITROGEN
ETHYLENE OXIDE + NITROGEN
![]() 20°C – 50°C
20°C – 50°C

cGMP ethylene oxide sterilisers
 PHARMA DIVISION
PHARMA DIVISION
cGMP ethylene oxide sterilisers
The ETO sterilisers have been designed to sterilise – by means of ethylene oxide – heat-sensitive materials, such as plastic syringes, infusion sets, dialysis filter cartridges, plastic materials and special surgical tools.
![]() POROUS AND NON-POROUS
POROUS AND NON-POROUS
![]() ETHYLENE OXIDE + NITROGEN
ETHYLENE OXIDE + NITROGEN
![]() 20°C – 50°C
20°C – 50°C
“Standard” and custom programmes for every need. he equipment process is developed by our Automation Department, according to the current codes/standards and the type of product being processed. Vacuum breaker or pressure leak tests; load conditioning programme; vacuum sterilisation programme; product degassing programme; and a gas neutralising programme, etc. The ETO gas is injected at a temperature of 60°C into the chamber via a PID-controlled modulating valve and the condensate is continuously evacuated through the drain, in order to ensure excellent heat distribution during the entire sterilisation phase (temperature deviation below ± 1°C).
- Double-walled square chamber, made entirely from 316L or 316Ti stainless steel.
- Ashlar-type total cavity comprising 304 or 316L/316Ti stainless steel.
- Piping made entirely from 316L stainless steel with sanitary
- fittings (tri-clamp ferrules and hygienic flanges).
- Any surfaces that come into contact with the product and process fluids are mechanically polished to a roughness level of less than 0.35 micron.
- Automatic horizontal sliding chamber doors.
- Pneumatically pressurised chamber door sealing gasket (cwith process air).
- 316L/316Ti stainless steel components and tools, in addition to elastomers manufactured in compliance with FDA 21 CFR, Part 177.
- The chamber, heads, pipes, components and instruments are properly insulated by a cutting-edge material.
- Built-in steam generator.
- Built-in vacuum pump with a water recirculation system (water-saving system).
- Built-in ETO gas evaporation system.
- Built-in load conditioning, sterilising and degassing devices.
- Manual or fully automated ergonomic product loading and unloading solutions.
- Gas neutralisation system by means of a thermal combustor or scrubber.
- Floor or elevated loading solutions.
We may be small, but we can achieve great things.
Thanks to our customisable after-sales service packages, we offer solutions to suit every need. Choose the one that suits you best!
Customisation is quick and easy
Keep up to date with the latest news from the Last Technology world
and let us steer you towards the most suitable service for you!